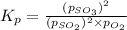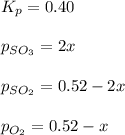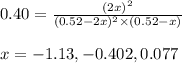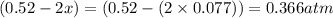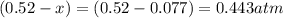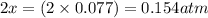Chemistry, 27.12.2019 03:31 IIHarmonyII
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of so2, o2, and so3 produced from an initial mixture in which the partial pressures of so2 and o2 = 0.52 atm and the partial pressure of so3 = 0 (exactly).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1



Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g)...
Questions in other subjects:

English, 13.07.2019 05:30




Chemistry, 13.07.2019 05:30




Health, 13.07.2019 05:30

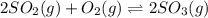
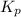 for above equation follows:
for above equation follows: