
Consider four different samples: aqueous licl, molten licl, aqueous agcl, and molten agcl. current run through each sample produces one of the following products at the cathode: solid lithium, solid silver, or hydrogen gas. match each sample to its cathodic product.
drag the appropriate items to their respective bins.
bin 1) solid lithium
bin 2) solid silver
bin 3) hydrogen gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Consider four different samples: aqueous licl, molten licl, aqueous agcl, and molten agcl. current...
Questions in other subjects:



Mathematics, 20.10.2019 19:50

Mathematics, 20.10.2019 19:50






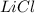

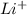 and
and  ions , and since its aqueous , it also contains
ions , and since its aqueous , it also contains  ions.But we know that , lithium is small in size and has a positive charge. Thus it has high polarizing power. Thus it cannot move easily through water. Even if it does , it reacts with
ions.But we know that , lithium is small in size and has a positive charge. Thus it has high polarizing power. Thus it cannot move easily through water. Even if it does , it reacts with  to form
to form 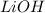 .
. ⇒
⇒
 ions. Thus the product at cathode would be :
ions. Thus the product at cathode would be : ⇒
⇒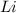
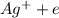 ⇒
⇒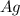 .....0.80V
.....0.80V ⇒
⇒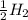 ....0.00V
....0.00V

