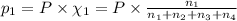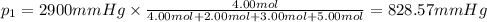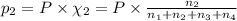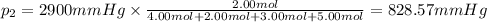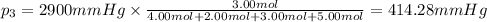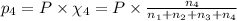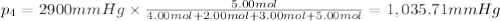Chemistry, 27.12.2019 02:31 ahmedeldyame
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles of ar. the total pressure of the mixture is 2900 mm. determine the mole fraction of each gas in the mixture. determine the mole percent of each gas in the mixture. determine the partial pressure of each gas in the mixture.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
You know the right answer?
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles o...
Questions in other subjects:


English, 31.08.2020 04:01


Chemistry, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01


History, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

History, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

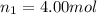
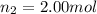
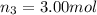
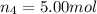
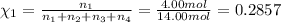
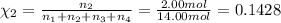
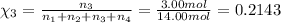

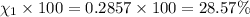

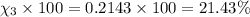
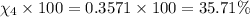
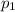
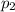

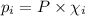
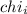 = Mole fraction of ith component
= Mole fraction of ith component