
Chemistry, 27.12.2019 00:31 lestessanders02
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following reaction equation: 2 h2s + 3 o2 → 2 so2 + 2 h2o

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3



Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following rea...
Questions in other subjects:


English, 12.02.2021 19:50


Mathematics, 12.02.2021 19:50


Social Studies, 12.02.2021 19:50




Mathematics, 12.02.2021 19:50

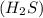 undergoes combustion to yield sulfur dioxide and water by the following reaction equation:
undergoes combustion to yield sulfur dioxide and water by the following reaction equation:
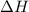 of the reaction if 26.2 g of
of the reaction if 26.2 g of  reacts with excess
reacts with excess 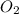 to yield 431.8 kJ?
to yield 431.8 kJ? 




