
Chemistry, 27.12.2019 00:31 ehuntsman8221
Asolution is prepared by dissolving 0.56 g of benzoic acid (c6h5co2h, ka 6.4 ) in enough water to make 1.0 l of solution. calculate [c6h5co2h], , , , and the ph of this solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Asolution is prepared by dissolving 0.56 g of benzoic acid (c6h5co2h, ka 6.4 ) in enough water to...
Questions in other subjects:

History, 02.08.2019 16:00


Social Studies, 02.08.2019 16:00


Computers and Technology, 02.08.2019 16:00


Computers and Technology, 02.08.2019 16:00

Chemistry, 02.08.2019 16:00

Chemistry, 02.08.2019 16:00

![$\left[\mathrm{H}^{+}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]=5.1 \times 10^{-4} \mathrm{M}$](/tpl/images/0434/1977/10f45.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=4.1 \times 10^{-3} \mathrm{M}$](/tpl/images/0434/1977/9a3e2.png)
![$\left[\mathrm{OH}^{-}\right]=1.9 \times 10^{-11} \mathrm{M}](/tpl/images/0434/1977/e5b4e.png)
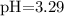
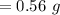
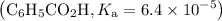
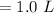
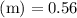
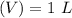
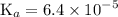
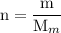
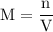
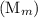 and the number of moles (n) of benzoic acid:
and the number of moles (n) of benzoic acid: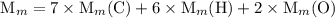
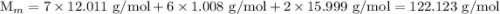
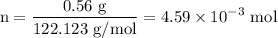
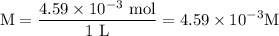
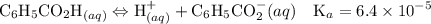
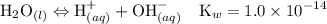
![$\mathrm{K}_{a}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]}{\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]}=6.4 \times 10^{-5}$](/tpl/images/0434/1977/e74c4.png)
![$\left[\mathrm{H}^{+}\right]_{0}$](/tpl/images/0434/1977/f38bd.png) is 0. (because of autonization)
is 0. (because of autonization)![$\left[\mathrm{H}^{+}\right]_{0}=10^{-7} \mathrm{M} \approx 0$](/tpl/images/0434/1977/a3a3d.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]_{0}-\mathrm{x}=0.00459-\mathrm{x}$](/tpl/images/0434/1977/a086a.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]_{0}+\mathrm{x}=0+\mathrm{x}=\mathrm{x}$](/tpl/images/0434/1977/89a6e.png)
![$\left[\mathrm{H}^{+}\right]=\left[\mathrm{H}^{+}\right]_{0}+x=0+x=x$](/tpl/images/0434/1977/2b64c.png)
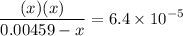
![$\left[\mathrm{H}^{+}\right]$](/tpl/images/0434/1977/93e23.png) is much smaller than
is much smaller than 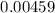 to decide whether the following approximation is valid or not:
to decide whether the following approximation is valid or not: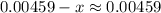
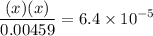
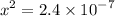
![$\mathrm{x}=4.9 \times 10^{-4}=\left[\mathrm{H}^{+}\right]$\\](/tpl/images/0434/1977/329c6.png)
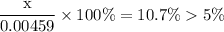
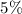 of acid is dissociated.
of acid is dissociated.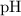 :
: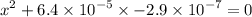
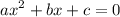
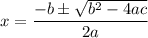
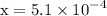
![\mathrm{M}=\left[\mathrm{H}^{+}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]$](/tpl/images/0434/1977/6835a.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=0.00459-0.00051=4.1 \times 10^{-3} \mathrm{M}$](/tpl/images/0434/1977/3ea5f.png)
![$\left[\mathrm{OH}^{-}\right]=\frac{\mathrm{K}_{w}}{\left[\mathrm{H}^{+}\right]}=\frac{1.0 \times 10^{-14}}{5.1 \times 10^{-4}}=1.9 \times 10^{-11} \mathrm{M}$](/tpl/images/0434/1977/7340a.png)
![$\mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]=-\log \left(5.1 \times 10^{-4}\right)=3.29$](/tpl/images/0434/1977/3ad18.png)


