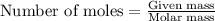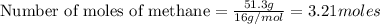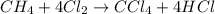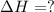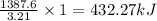Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Methane (ch4) reacts with cl2 to yield ccl4 and hcl by the following reaction equation: ch4 + 4 cl2...
Questions in other subjects:

History, 17.12.2020 21:00





Biology, 17.12.2020 21:00


Social Studies, 17.12.2020 21:00


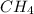 reacts with excess
reacts with excess 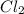 to yield 1387.6 kJ is 432.27kJ
to yield 1387.6 kJ is 432.27kJ