
Chemistry, 26.12.2019 23:31 coryowens44
1. for a hydrogen‑like atom, classify the electron transitions according to whether they result in the absorption or emission of light? n=1 to n=3, n=2 to n=1, n=3 to n=2, n=3 to n=52. ignoring sign, which transition is associated with the greatest energy change? o n=1 to n=3 o n=2 to n=1 o n=3 to n=2 o n=3 to n=5

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

You know the right answer?
1. for a hydrogen‑like atom, classify the electron transitions according to whether they result in t...
Questions in other subjects:


Mathematics, 16.02.2021 22:10


Mathematics, 16.02.2021 22:10


Biology, 16.02.2021 22:10

Physics, 16.02.2021 22:10

Mathematics, 16.02.2021 22:10



 = energy of
= energy of 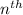 orbit
orbit

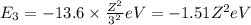

 (absorption)
(absorption) (emission)
(emission) (emission)
(emission)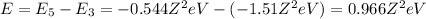 (absorption)
(absorption)

