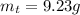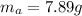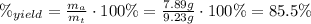Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
Areaction with a theoretical yield of 9.23 g produced 7.89 g of product. what is the percent yield f...
Questions in other subjects:

History, 11.11.2021 01:00

Mathematics, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00

Health, 11.11.2021 01:00

Chemistry, 11.11.2021 01:00



Mathematics, 11.11.2021 01:00