
Chemistry, 26.12.2019 22:31 DavidsonSaid
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4.4 m solution of boric acid. round your answer to 1 decimal place.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

You know the right answer?
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4...
Questions in other subjects:

Mathematics, 21.05.2020 22:17


English, 21.05.2020 22:17


Mathematics, 21.05.2020 22:17




Mathematics, 21.05.2020 22:17

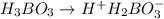
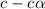
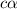
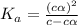
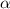 = ?
= ?
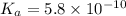
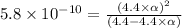
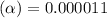
![[H^+]=c\times \alpha](/tpl/images/0434/0871/4fc41.png)
![[H^+]=4.4\times 0.000011=4.8\times 10^{-5}M](/tpl/images/0434/0871/865ab.png)
![pH=-log[H^+]](/tpl/images/0434/0871/15713.png)
![pH=-log[4.8\times 10^{-5}]=4.3](/tpl/images/0434/0871/ee066.png)
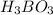 solution is 4.3
solution is 4.3


