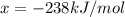Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Consider the following reaction: a2 + b2 → 2ab δh = –321 kj bond energy (a2) = 1/2ab bond energy (b...
Questions in other subjects:

English, 28.01.2021 18:10


Mathematics, 28.01.2021 18:10

English, 28.01.2021 18:10

Biology, 28.01.2021 18:10





Mathematics, 28.01.2021 18:10

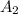 is -238 kJ/mol
is -238 kJ/mol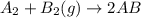

![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0434/0215/42942.png)
![\Delta H=[(n_{A_2}\times B.E_{A_2})+(n_{B_2}\times B.E_{B_2}) ]-[(n_{AB}\times B.E_{AB})]](/tpl/images/0434/0215/c021c.png)
![\Delta H=[(1\times x)+(1\times B.E_{B_2}) ]-[(2\times 2x)]](/tpl/images/0434/0215/7aa1f.png)
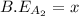
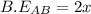
![-321=[(1\times x)+(1\times 393)]-[(2\times 2x)]](/tpl/images/0434/0215/06819.png)