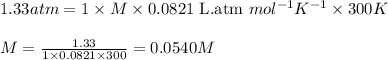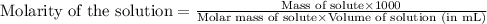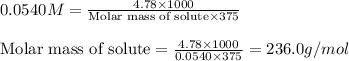Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

You know the right answer?
Asolution is prepared by dissolving 4.78 g of an unknown nonelectrolyte in enough water to make 375...
Questions in other subjects:

English, 27.08.2019 13:10

History, 27.08.2019 13:10

Spanish, 27.08.2019 13:10




Physics, 27.08.2019 13:10


Mathematics, 27.08.2019 13:10

Mathematics, 27.08.2019 13:10

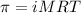
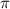 = osmotic pressure of the solution = 1.33 atm
= osmotic pressure of the solution = 1.33 atm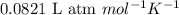
![27^oC=[273+27]K=300K](/tpl/images/0434/0272/00f96.png)