
Chemistry, 25.12.2019 23:31 spacehunter22
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration was found to be 4.102. if the initial ph of the weak acid solution (before titration) has a ph of 2.308, what was the concentration of the weak acid solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration...
Questions in other subjects:

Mathematics, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00


Mathematics, 18.05.2021 14:00


Social Studies, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00

Business, 18.05.2021 14:00

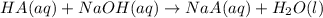
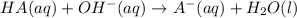
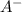 produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:
produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:![pH = pK_a + log(\frac{[A^-]}{[HA})](/tpl/images/0433/2346/a2dd4.png)
![[HA] = [A^-]](/tpl/images/0433/2346/96807.png) when half of equivalence volume of NaOH is added.
when half of equivalence volume of NaOH is added. at midpoint. We may then find the acid ionization constant for this acid:
at midpoint. We may then find the acid ionization constant for this acid:
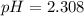
![pH = -log[H_3O^+]](/tpl/images/0433/2346/89cf7.png) , then
, then ![[H_3O^+] = 10^{-pH}](/tpl/images/0433/2346/341c1.png)
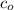 , we may write the equilibrium constant expression as:
, we may write the equilibrium constant expression as:![K_a = \frac{[H_3O^+]^2}{c_o - [H_3O^+]}](/tpl/images/0433/2346/86a10.png)
![c_o = \frac{[H_3O^+]^2}{K_a} + [H_3O^+] = \frac{10^{-2pH}}{K_a} + 10^{-pH} = \frac{10^{-2\cdot 2.308}}{7.91\cdot 10^{-5}} + 10^{-2.308} = 0.311 M](/tpl/images/0433/2346/5826b.png)


