
Chemistry, 25.12.2019 03:31 apowers6361
Select the substance in each pair that should have the higher boiling point. in each case identify the principal intermolecular forces involved and account briefly for your choice.
(a) k2s or (ch3)3n,
(b) br2 or ch3ch2ch2ch3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, petriajack8375
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3


Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Select the substance in each pair that should have the higher boiling point. in each case identify t...
Questions in other subjects:


Advanced Placement (AP), 26.06.2019 21:10

Mathematics, 26.06.2019 21:10

History, 26.06.2019 21:10


Mathematics, 26.06.2019 21:10



Mathematics, 26.06.2019 21:10

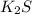 (b)
(b) 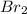
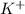 and
and  ions are tightly bound together through ion-ion attraction force (principal intermolecular force).
ions are tightly bound together through ion-ion attraction force (principal intermolecular force).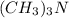 is a polar covalent molecule. So dipole-dipole attraction force (principal intermolecular force) exists in this molecule.
is a polar covalent molecule. So dipole-dipole attraction force (principal intermolecular force) exists in this molecule.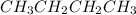 are nonpolar covalent compounds. Hence only london dispersion force(principal intermolecular force) exists in both molecules.
are nonpolar covalent compounds. Hence only london dispersion force(principal intermolecular force) exists in both molecules.


