
Chemistry, 25.12.2019 01:31 lauralimon
The bomb calorimeter in exercise 102 is filled with 987g water. the initial temperature of the calorimeter contents is 23.32. a 1.056g sample of benzoic acid is combusted in the calorimeter. what is the final temperature of the calorimeter contents

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

You know the right answer?
The bomb calorimeter in exercise 102 is filled with 987g water. the initial temperature of the calor...
Questions in other subjects:



Mathematics, 07.07.2019 17:00




Social Studies, 07.07.2019 17:00

Social Studies, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

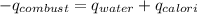
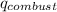 = heat of combustion of benzoic acid
= heat of combustion of benzoic acid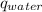 = heat energy released to water
= heat energy released to water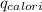 = heat energy released to the calorimeter
= heat energy released to the calorimeter![-m_{combust}*H_{combust} = [m_{water}*c_{water} + C_{calori}]*(T_{f} - T_{i})](/tpl/images/0432/3297/c7fd9.png)
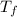 - 23.32)
- 23.32)

