
Chemistry, 25.12.2019 01:31 boneyke3720
The clectron in a hydrogen atom, originally in level n=9, undergoes a transition to a lower level by emitting a photon
of wavelength 384 nm. what is the final level of the electron?
c = 3.00 x 10 m/s,
h = 6.626 x 10^-34j-s,
rh= 2.179 x 10^-18

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
The clectron in a hydrogen atom, originally in level n=9, undergoes a transition to a lower level by...
Questions in other subjects:

Chemistry, 05.03.2021 03:30



Mathematics, 05.03.2021 03:30





Mathematics, 05.03.2021 03:30

 = 9,
= 9,  = ?
= ?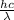
 Js
Js m/s
m/s = wavelength
= wavelength ![\Delta E = -2.179 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0432/3307/57fbb.png)
![-2.179 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0432/3307/1f28d.png)
 =
= ![-2.179 \times 10^{-18} J \times (1)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{(9)^{2}}]](/tpl/images/0432/3307/e4713.png)



