Chemistry, 25.12.2019 01:31 zeesharpe05
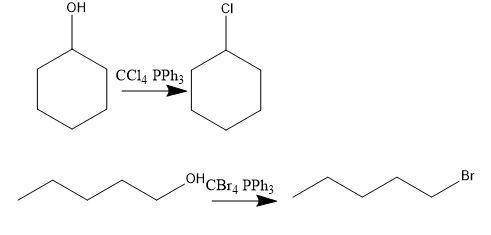
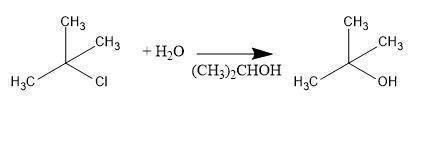
Consider the above reaction using 25.0 ml of tert-butyl alcohol (d = 0.786 g/ml) with 60.0 ml of concentrated hydrobromic acid (d = 1.49 g/ml, 47.0% hbr). on a separate sheet calculate the theoretical yield in grams and the percent yield for a reaction that produced 26.1 g of tert-butyl bromide. clearly show the set ups to determine the limiting reactant and other calculations using proper units and significant figures. consult your textbook for the following two synthesis. keep in mind that conc. hcl and conc. hbr will not give good yields of alkyl halides by the reaction with primary and secondary alcohols. give the balanced equation to prepare chlorocyclohexane in good yield from cyclohexanol. give the balanced equation for the preparation in good yield of 1-bromopentane from 1-pentanol. the reverse of the reaction you performed in the lab can also occur. under the proper conditions, tertiary alkyl halides may undergo a hydrolysis reaction to form an alcohol and a hydrogen halide. complete and balance the following equation. (remember the general ii experiment? )

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:40, alexandraparava
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 13:10, Jana1517
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes. which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature. sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1
You know the right answer?
Consider the above reaction using 25.0 ml of tert-butyl alcohol (d = 0.786 g/ml) with 60.0 ml of con...
Questions in other subjects:

Mathematics, 09.03.2021 16:30




Health, 09.03.2021 16:30

Mathematics, 09.03.2021 16:30

Mathematics, 09.03.2021 16:30

Spanish, 09.03.2021 16:30