
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285 g) and h20 (0.658g) are produced. the mol mass of the compound is determined by mass spectrometry to be 90 g/mole determine the empirical and molecular formulas. 20: 0-8453x(12.01s /uu. o 032 029/1b. a mal 1 2

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

You know the right answer?
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285...
Questions in other subjects:




Mathematics, 16.12.2020 21:50

Biology, 16.12.2020 21:50


Mathematics, 16.12.2020 21:50

Spanish, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50

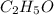
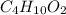
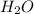 = 0.658 g /18 g/mol = 0.03656 moles
= 0.658 g /18 g/mol = 0.03656 moles
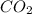 = 1.285 g
/44.01 g/mol = 0.029197 moles
= 1.285 g
/44.01 g/mol = 0.029197 moles


