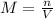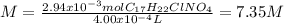Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
The hydrochloride form of cocaine has a solubility of 1.00 g in 0.400 ml water. calculate the molari...
Questions in other subjects:





Mathematics, 06.06.2020 04:00

Mathematics, 06.06.2020 04:00

History, 06.06.2020 04:00