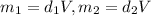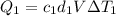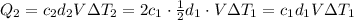You have two perfectly insulated cups. one contains water and the other contains an equal volume of another liquid that has half the density of water and twice the specific heat capacity. you heat the water from 10ºc to 20ºc and other liquid from 80ºc to 90ºc. compare the amount of heat energy needed to raise the temperature of the other liquid to the amount needed to raise the temperature of the water.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
You have two perfectly insulated cups. one contains water and the other contains an equal volume of...
Questions in other subjects:


Mathematics, 21.12.2019 22:31

Mathematics, 21.12.2019 22:31



History, 21.12.2019 22:31




Mathematics, 21.12.2019 22:31

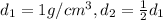 ;
; ;
;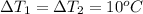 ;
;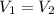 .
.