Chemistry, 24.12.2019 03:31 jaleewoodyard1
An analytical chemist is titrating 179.5ml of 0.8600ma solution of cyanic acid(hcno) with0.6500m a solution of naoh. the pka of cyanic acid is 3.46 . calculate the ph of the acid solution after the chemist has added 274.6ml of naoh the solution to it.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
An analytical chemist is titrating 179.5ml of 0.8600ma solution of cyanic acid(hcno) with0.6500m a s...
Questions in other subjects:










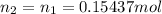
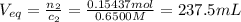
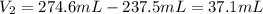
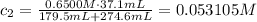
![pOH = -log[NaOH] = -log(0.053105) = 1.27](/tpl/images/0431/2587/d1ce5.png)