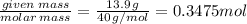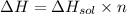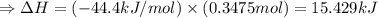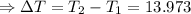Chemistry, 24.12.2019 02:31 coolman5999alt
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves in 250.0 g of water 23.0 °c in a coffee-cup calorimeter, what is the final temperature of the solution assuming no heat is lost to the surroundings. the solution has the same specific heat of 4.184 j/g-k.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1



Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves...
Questions in other subjects:

Mathematics, 15.09.2019 04:10



English, 15.09.2019 04:10


Mathematics, 15.09.2019 04:10




 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,