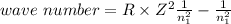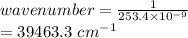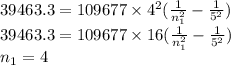Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 13:00, kayleegeise
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
One of the emission spectral lines for be3+ has a wavelength of 253.4 nm for an electronic transitio...
Questions in other subjects:

Mathematics, 17.06.2020 16:57

Mathematics, 17.06.2020 16:57


Mathematics, 17.06.2020 16:57

Spanish, 17.06.2020 16:57