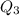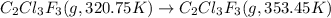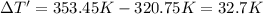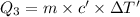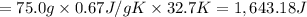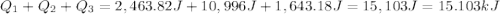Chemistry, 23.12.2019 21:31 lacyfigueroa
The fluorocarbon compound c2cl3f3 has a normal boiling point of 47.6 °c. the specific heats of c2cl3f3(l) and c2cl3f3(g) are 0.91 j/g. k and 0.67 j/g. k, respectively. the heat of vaporization for the compound is 27.49 kj/mol.
calculate the heat required to convert 75.0 g of c2cl3f3 from a liquid at 11.50 °c to a gas at 80.30 °c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1


Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
The fluorocarbon compound c2cl3f3 has a normal boiling point of 47.6 °c. the specific heats of c2cl3...
Questions in other subjects:


Mathematics, 05.01.2021 17:00






History, 05.01.2021 17:00


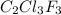 from a liquid at 11.50 °C to a gas at 80.30 °C is 15.103 kiloJoules.
from a liquid at 11.50 °C to a gas at 80.30 °C is 15.103 kiloJoules.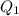
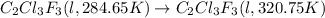
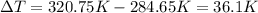
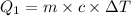
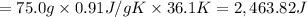
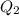
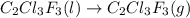
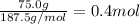
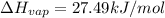
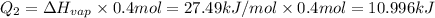
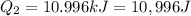 (1 kJ= 1000 J)
(1 kJ= 1000 J)