Chemistry, 23.12.2019 17:31 akatherine1738
Mothballs are composed primarily of the hydrocarbon naphthalene (c10h8). when 1.025 g of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘c to 32.33 ∘c. find δerxn for the combustion of naphthalene. the heat capacity of the calorimeter, determined in a separate experiment, is 5.11kj/∘c.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1


Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (c10h8). when 1.025 g of naphthalene...
Questions in other subjects:





Mathematics, 09.03.2020 04:58


Mathematics, 09.03.2020 04:59



 for combustion of naphthalene is -5164 kJ/mol
for combustion of naphthalene is -5164 kJ/mol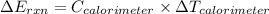
 refers change in temperature.
refers change in temperature.

 of naphthalene
of naphthalene