Chemistry, 20.12.2019 21:31 petroale000
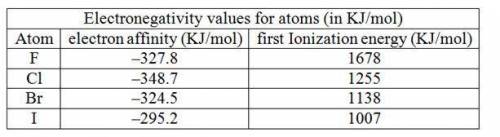
An alternative definition of electronegativity is electronegativity= constant (i. e — e. a.) where i. e. is the ionization energy and e. a. is the electron affinity using the sign conventions of this book. use data in chapter 12 to calculate the (i. e. - e. a.) term for f, cl, br, and i. do these values show the same trend as the electronegativity values given in this chapter? the first ionization energies of the halogens are 1678, 1255, 1138, and 1007 kj/mol, respectively.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 06:50, isabellainksow87vn
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
An alternative definition of electronegativity is electronegativity= constant (i. e — e. a.) where i...
Questions in other subjects:



Mathematics, 27.06.2019 23:30


Health, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30




Social Studies, 27.06.2019 23:30

![4=constant[1678-(-327.8)]](/tpl/images/0428/1473/7fe37.png)
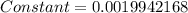
![Electronegativity=0.0019942168[1255+348.7]=3.1980\sim 3](/tpl/images/0428/1473/aa358.png)
![Electronegativity=0.0019942168[1138+324.5]=2.91\sim 2.9](/tpl/images/0428/1473/6b430.png)
![Electronegativity=0.0019942168[1007+295.7]=2.59\sim 2.5](/tpl/images/0428/1473/4bd84.png)