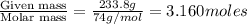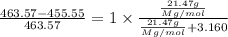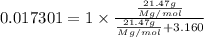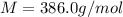Chemistry, 20.12.2019 21:31 cindy14772
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. the vapor pressure of diethyl ether, ch3ch2och2ch3, is 463.57 mm hg at 25°c.
1. in a laboratory experiment, students synthesized a new compound and found that when 21.47 grams of the compound were dissolved in 233.8 grams of diethyl ether, the vapor pressure of the solution was 455.55 mm hg. the compound was also found to be nonvolatile and a non-electrolyte. what is the molecular weight of this compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in...
Questions in other subjects:











 = relative lowering in vapor pressure
= relative lowering in vapor pressure = mole fraction of solute =
= mole fraction of solute =