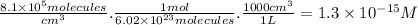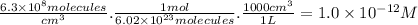The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6 x 10^8 m^-1s^-1 at 4°c.
part a) if the tropospheric concentrations of oh and cf3ch2f are 8.1 x 10^5 and 6.3 x 10^8 molecules/cm^3, respectively, what is the rate of reaction at this temperature in m/s?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, creepycrepes
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2


Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 06:30, lwattsstudent
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1
You know the right answer?
The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactan...
Questions in other subjects:


Physics, 15.11.2019 20:31



History, 15.11.2019 20:31

Health, 15.11.2019 20:31



Spanish, 15.11.2019 20:31

Mathematics, 15.11.2019 20:31