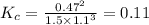Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions in other subjects:

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00



Mathematics, 30.01.2021 01:00


Mathematics, 30.01.2021 01:00


Mathematics, 30.01.2021 01:00

Geography, 30.01.2021 01:00

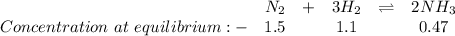
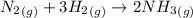
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0428/1906/c3aa0.png)