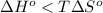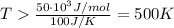Chemistry, 20.12.2019 20:31 mokunola16
At what temperature would a given reaction become spontaneous if h = kj and s = j/k? the instructor will choose a value for delta h between 50 kj and 250 kj and delta s between 100 j/k and 300 j/k. the correct answer must show the relationship between delta h and delta s that is used to calculate the temperature at which the change occurs as well as the calculations themselves. the final answer must have the correct units with it. any answer that simply shows a numerical value with units will not be accepted.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
At what temperature would a given reaction become spontaneous if h = kj and s = j/k? the instruct...
Questions in other subjects:





Mathematics, 25.09.2019 05:30

English, 25.09.2019 05:30


English, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30

Business, 25.09.2019 05:30


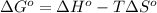 ;if the change in the Gibbs free energy is negative, the reaction is spontaneous, that is:
;if the change in the Gibbs free energy is negative, the reaction is spontaneous, that is: 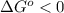 or
or 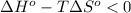 ;if the change in the Gibbs free energy is positive, the reaction is non-spontaneous, that is:
;if the change in the Gibbs free energy is positive, the reaction is non-spontaneous, that is: 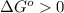 or
or 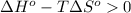 .
.