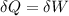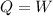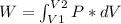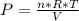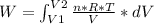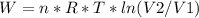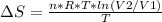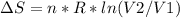Chemistry, 20.12.2019 19:31 Mattixwillard
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally reversible process.
(a) determine if the entropy change of the gas is greater than, equal to or less than zero, justify your answer
(b) determine if for the same change of state, the entropy change for an irreversible process is greater than, equal to or less than part (a)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
You know the right answer?
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally rev...
Questions in other subjects:

Mathematics, 05.05.2020 05:29

Mathematics, 05.05.2020 05:29



Geography, 05.05.2020 05:29




Mathematics, 05.05.2020 05:29


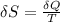
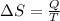
![\delta U=[tex]\delta Q- \delta W](/tpl/images/0427/9845/795a2.png)
![0=[tex]\delta Q- \delta W](/tpl/images/0427/9845/24655.png)