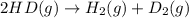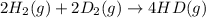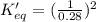The equilibrium constant is given for one of the reactions below.
determine the value o...

Chemistry, 20.12.2019 19:31 kayleedavis08
The equilibrium constant is given for one of the reactions below.
determine the value of the missing equilibrium constant.
2 hd(g) ⇌ h2(g) + d2(g) kc = 0.28
2 h2(g) + 2 d2(g) ⇌ 4 hd(g)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Questions in other subjects:









Mathematics, 01.02.2021 20:50

English, 01.02.2021 20:50