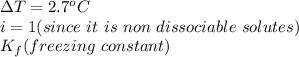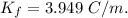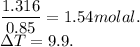Chemistry, 20.12.2019 19:31 savyblue1724707
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezing point of the solution is 2.7°c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh4ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x.
a) calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezi...
Questions in other subjects:

Mathematics, 13.12.2019 03:31



Mathematics, 13.12.2019 03:31


Mathematics, 13.12.2019 03:31

Mathematics, 13.12.2019 03:31




 .....1
.....1