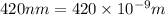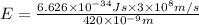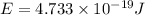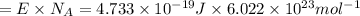Chemistry, 20.12.2019 18:31 pablogonzaleztellez
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 + hv > > > no(g) + o(g) the maximum wavelength of light that can cause this reaction is 420 nm. a) in what part of the electromagnetic spectrum is light with this wavelength found? b) what is the maximum strength of a bond, in kj/mol, that can be broken by absorption of a photon of 420-nm light? c) write out the photodissociation reaction showing lewis-dot structures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lizbeth232001
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 +...
Questions in other subjects:



Mathematics, 27.01.2021 19:10

English, 27.01.2021 19:10

Mathematics, 27.01.2021 19:10

Health, 27.01.2021 19:10

Chemistry, 27.01.2021 19:10

Mathematics, 27.01.2021 19:10


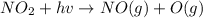
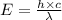
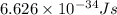
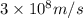
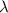 = wavelength =
= wavelength =