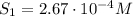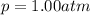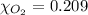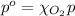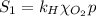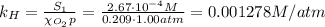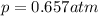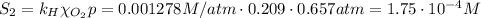The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the solubility of o2 from the air is 2.67 ✕ 10-4 m at sea level and 25°c, what is the solubility of o2 at an elevation of 12,000 ft where the atmospheric pressure is 0.657 atm? assume the temperature is 25°c, and that the mole fraction of o2 in air is 0.209 at both 12,000 ft and at sea level.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
You know the right answer?
The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the so...
Questions in other subjects:

Mathematics, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00


Geography, 07.07.2019 23:00

Mathematics, 07.07.2019 23:00

English, 07.07.2019 23:00

English, 07.07.2019 23:00

World Languages, 07.07.2019 23:00

Biology, 07.07.2019 23:00

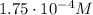
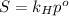
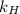 is Henry's law constant.
is Henry's law constant.