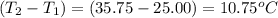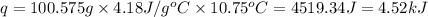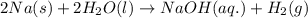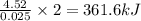Chemistry, 20.12.2019 18:31 King1Gates
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemical equation shown below.
when 0.0 25 mol of na is added to 100.00 g of water, the temperature of the resulting solution rises from 25.00°c to 35.75°c.
if the specific heat of the solution is 4.18 j/(g · °c), calculate δh for the reaction, as written.
2 na(s) + 2 h2o(l) → 2 naoh(aq) + h2(g) δh= ?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemica...
Questions in other subjects:





Biology, 12.03.2020 21:31

Mathematics, 12.03.2020 21:31


English, 12.03.2020 21:31


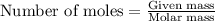
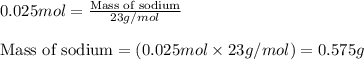
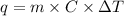
 = change in temperature =
= change in temperature =