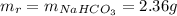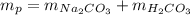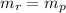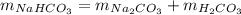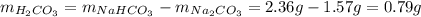10. a 2.36-gram sample of nahco3 was completely decomposed in an
experiment.
2nahco3 --...

10. a 2.36-gram sample of nahco3 was completely decomposed in an
experiment.
2nahco3 -- na2co3 + h2co3
in this experiment, carbon dioxide and water vapors combine to form
h2co3. after decomposition, the na2co3 had a mass of 1.57 grams.
determine the mass of the h2co3 produced.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 20:30, jaejaeJae9534
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Questions in other subjects:



English, 25.09.2021 22:20

Mathematics, 25.09.2021 22:20




Mathematics, 25.09.2021 22:20

Biology, 25.09.2021 22:20

History, 25.09.2021 22:20