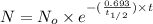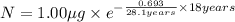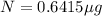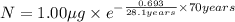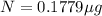Chemistry, 20.12.2019 06:31 bryan12376
One of the hazards of nuclear explosions is the generation of 90sr andits subsequent incorporation in place of calcium in bones. this nuclide emitsβrays of energy 0.55 mev, and has a half-life of 28.1 y. suppose 1.00 μg wasabsorbed by a newly born child. how much will remain after (a) 18 y, (b) 70 yif none is lost metabolically?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
One of the hazards of nuclear explosions is the generation of 90sr andits subsequent incorporation i...
Questions in other subjects:

Chemistry, 02.02.2021 05:10






Biology, 02.02.2021 05:10


Mathematics, 02.02.2021 05:10

History, 02.02.2021 05:10

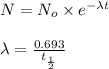
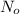 = initial mass of isotope
= initial mass of isotope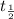 = half life of the isotope = 28.1 years
= half life of the isotope = 28.1 years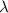 = rate constant
= rate constant