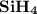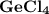1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
use the octet rule to predict the formula of the compound that would form between silicon and hydrogen , if the molecule contains only one silicon atom and only single bonds are formed.
formula:
2. the element germanium would be expected to covalent bond(s) in order to obey the octet rule. use the octet rule to predict the formula of the compound that would form between germanium and chlorine , if the molecule contains only one germanium atom and only single bonds are formed.
formula:

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3


Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
<...
<...
Questions in other subjects:



Mathematics, 01.02.2020 06:42

Mathematics, 01.02.2020 06:42

English, 01.02.2020 06:42

Biology, 01.02.2020 06:42




History, 01.02.2020 06:42