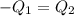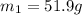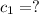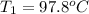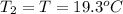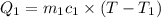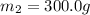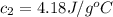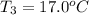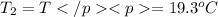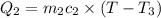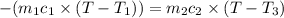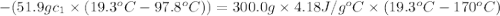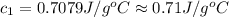Chemistry, 20.12.2019 05:31 nihadsalim10
A51.9 g sample of quartz is put into a calorimeter (see sketch at right) that contains 300.0 g of water. the quartz sample starts off at 97.8 °c and the temperature of the water starts off at 17.0 °c. when the temperature of the water stops changing it's 19.3 °c. the pressure remains constant at 1 atm. insulated container water sample calculate the specific heat capacity of quartz according to this experiment. be sure your answer is rounded to 2 significant digits. a calorimeter g °c.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1


Chemistry, 23.06.2019 04:00, Mitchmorgan3816
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
A51.9 g sample of quartz is put into a calorimeter (see sketch at right) that contains 300.0 g of wa...
Questions in other subjects:

Chemistry, 22.05.2021 04:00

Mathematics, 22.05.2021 04:00

Mathematics, 22.05.2021 04:00

Mathematics, 22.05.2021 04:00

History, 22.05.2021 04:00

Mathematics, 22.05.2021 04:00

Mathematics, 22.05.2021 04:00



Mathematics, 22.05.2021 04:00