
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in step 2, it releases 5j of heat at 1 atm. as it returned to its original internal energy. find the change in the volume of the system during the second step and identify it as an expansion or compression.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2


Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Asystem undergoes a two-step process. in step 1, it absorbs 50. j of heat at constant volume. in ste...
Questions in other subjects:



Law, 28.11.2019 21:31

Computers and Technology, 28.11.2019 21:31


World Languages, 28.11.2019 21:31


Business, 28.11.2019 21:31

World Languages, 28.11.2019 21:31

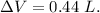
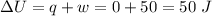
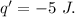
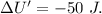
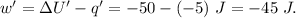
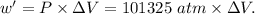
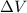 is positive.
is positive.


