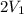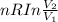Chemistry, 20.12.2019 03:31 arifkarimi9214
Calculate the change in the entropy of the system and also the change in the entropy of the surroundings, and the resulting total change in entropy, when a sample of nitrogen gas of mass 14 g at 298 k and 1.00 bar doubles its volume in (a) an isothermal reversible expansion, (b) an isothermal irreversible expansion against pext = 0, and (c) an adiabatic reversible expansion.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, Brittpaulina
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Calculate the change in the entropy of the system and also the change in the entropy of the surround...
Questions in other subjects:

Physics, 03.11.2020 23:00

Mathematics, 03.11.2020 23:00

Mathematics, 03.11.2020 23:00


Business, 03.11.2020 23:00

Mathematics, 03.11.2020 23:00

Biology, 03.11.2020 23:00

Biology, 03.11.2020 23:00

Mathematics, 03.11.2020 23:00

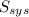 = 2.881 J/K; Δ
= 2.881 J/K; Δ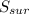 = -2.881 J/K; total change in entropy = 0
= -2.881 J/K; total change in entropy = 0 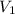
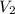 =
=