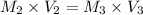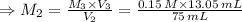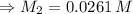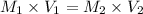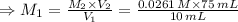Chemistry, 20.12.2019 02:31 halimomohamed
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer flask. an excess of ki solution was added. indicator was added and the solution was diluted with h2o to a total volume of 75 ml. for rxn 2, the solution from rxn 1 was titrated with 0.15 m na2s2o3. the equivalence point of the titration was reached when 13.05 ml of na2s2o3 had been added. what is the molar concentration of cu2+ in the original 10.0 ml solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer fla...
Questions in other subjects:

Mathematics, 01.12.2020 08:10

English, 01.12.2020 08:10


English, 01.12.2020 08:10

History, 01.12.2020 08:10


Biology, 01.12.2020 08:20

Mathematics, 01.12.2020 08:20

Chemistry, 01.12.2020 08:20

Mathematics, 01.12.2020 08:20