
Chemistry, 20.12.2019 02:31 ameera1973
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calcium hydroxide are added to this system, indicate whether the following statements are true or false. (assume that the volume does not change upon the addition of calcium hydroxide.)
a. the number of moles of ch3cooh will increase.
b. the number of moles of ch3coo- will decrease.
c. the equilibrium concentration of h3o will remain the same.
d. the ph will decrease.
e. the ratio of [ch3cooh] / [ch3coo-] will increase.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
1.00 liter solution contains 0.40 m acetic acid and 0.52 m potassium acetate. if 0.130 moles of calc...
Questions in other subjects:



English, 01.03.2021 08:40

Mathematics, 01.03.2021 08:40



History, 01.03.2021 08:40

English, 01.03.2021 08:40

Mathematics, 01.03.2021 08:40

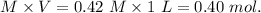
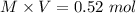


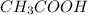 decrease and
decrease and 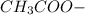 increase.
increase.

