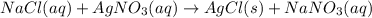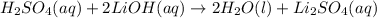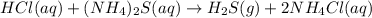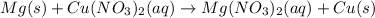Chemistry, 20.12.2019 02:31 wyattgrubb00
Nacl(aq) agno3(aq) → agcl(s) nano3(aq) : nacl(aq) agno3(aq) \rightarrow agcl(s) nano3(aq) : blank h2so4(aq) 2 lioh(aq) → 2 h2o(l) li2so4(aq) : h2so4(aq) 2 lioh(aq) \rightarrow 2 h2o(l) li2so4(aq) : blank hcl(aq) (nh4)2s(aq) → h2s(g) 2 nh4cl(aq) : hcl(aq) (nh4)2s(aq) \rightarrow h2s(g) 2 nh4cl(aq) : blank mg(s) cu(no3)2(aq) → mg(no3)2(aq) cu(s) :

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 16:00, esme06quirino
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
Nacl(aq) agno3(aq) → agcl(s) nano3(aq) : nacl(aq) agno3(aq) \rightarrow agcl(s) nano3(aq) : blank...
Questions in other subjects:



Biology, 15.09.2021 15:30





Health, 15.09.2021 15:30


Mathematics, 15.09.2021 15:30