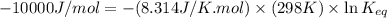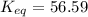Chemistry, 20.12.2019 01:31 maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions in other subjects:

Biology, 06.10.2019 07:40


Mathematics, 06.10.2019 07:40

History, 06.10.2019 07:40

Chemistry, 06.10.2019 07:40


English, 06.10.2019 07:40



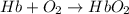 ;
; 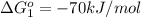
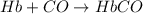 ;
; 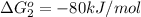
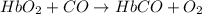 ;
; 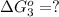
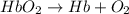 ;
; 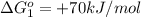
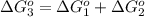
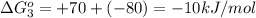
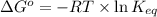
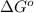 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol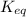 = equilibrium constant = ?
= equilibrium constant = ?