
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd(s) 2 ag(s) + cd2+ (a) voltage increases. (b) voltage decreases but remains > zero. (c) voltage becomes zero and remains at zero. (d) no change in voltage occurs. (e) direction of voltage change cannot be predicted without additional information. which of the above occurs for each of the following circumstances? 14. a 50-milliliter sample of a 2-molar cd(no3)2 solution is added to the left beaker. 15. the silver electrode is made larger. 16. the salt bridge is replaced by a platinum wire. 17. current is allowed to flow for 5 minutes.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd...
Questions in other subjects:

Mathematics, 07.06.2021 22:40

Engineering, 07.06.2021 22:40

Computers and Technology, 07.06.2021 22:40

Mathematics, 07.06.2021 22:40

Mathematics, 07.06.2021 22:40



Mathematics, 07.06.2021 22:40

History, 07.06.2021 22:40

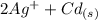 ⇒
⇒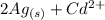
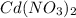 solution is added to the left beaker.
solution is added to the left beaker. 

