Chemistry, 19.12.2019 23:31 akatsionis25
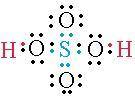
Draw the lewis structure for h2so4 (h is bonded to o). draw the molecule by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons. include all hydrogen atoms. to change the symbol of an atom, double-click on the atom and enter the letter of the new atom.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Draw the lewis structure for h2so4 (h is bonded to o). draw the molecule by placing atoms on the gri...
Questions in other subjects:


Mathematics, 28.05.2021 01:00