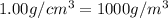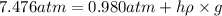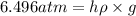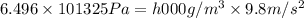Chemistry, 19.12.2019 23:31 hosteenimport21
The volume of a bubble that starts at the bottom of a lake at 4.55°c increases by a factor of 8.00 as it rises to the surface where the temperature is 18.05°c and the air pressure is 0.980 atm. assuming that the density of the lake water is 1.00 g/cm3, determine the depth of the lake?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
The volume of a bubble that starts at the bottom of a lake at 4.55°c increases by a factor of 8.00 a...
Questions in other subjects:

Mathematics, 26.02.2021 14:00

Social Studies, 26.02.2021 14:00

History, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00


Mathematics, 26.02.2021 14:00

Arts, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

Arts, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

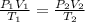
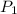 = initial pressure of gas in bubble= ?
= initial pressure of gas in bubble= ?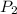 = final pressure of gas = 0.980 atm
= final pressure of gas = 0.980 atm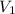 = initial volume of gas =
= initial volume of gas = 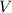
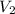 = final volume of gas = 8.00 × V
= final volume of gas = 8.00 × V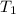 = initial temperature of gas =
= initial temperature of gas = 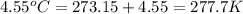
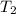 = final temperature of gas =
= final temperature of gas = 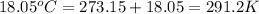
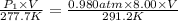
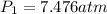
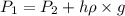
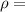 density of water =
density of water =