Chemistry, 19.12.2019 22:31 keilyjaramillo2870
Consider the reaction: m + 2hcl → mcl2 + h2 when 0.85 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 4035 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? ) it is possible that your answer could be either positive or negative. if it is negative, you must include the "minus" sign. enter your answer as a decimal number.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
Consider the reaction: m + 2hcl → mcl2 + h2 when 0.85 mol of the metal, m, reacted with an aqueous...
Questions in other subjects:

Computers and Technology, 26.02.2021 20:40

History, 26.02.2021 20:40


Mathematics, 26.02.2021 20:40

Mathematics, 26.02.2021 20:40


English, 26.02.2021 20:40


Mathematics, 26.02.2021 20:40


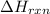 = enthalpy change of the reaction
= enthalpy change of the reaction