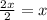In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2. what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy half of the tetrahedral holes? ab a3b ab2 a2b what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the tetrahedral holes? ab a2b ab2 a3b

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions in other subjects:

Physics, 28.07.2019 00:00




Mathematics, 28.07.2019 00:00


Mathematics, 28.07.2019 00:00


English, 28.07.2019 00:00